https://medium.com/microbial-instincts/cancer-ms-atrial-fibrillation-macular-degeneration-parkinsons-quantum-biology7f27d4f110ba-7f27d4f110ba
“Electron tunneling through ferritin (a blood protein that contains iron)
One of the overlooked quantum biological phenomena is electron tunneling associated with ferritin, an iron storage protein.
Tunneling is the “spooky” ability of a particle, such as an electron or proton, to move across a barrier, typically an unoccupied space. While there is evidence of electron and proton tunneling associated with proteins and enzymatic reactions, tunneling has to be inferred from other data and cannot be directly observed. At the scale of atoms and molecules, that data is usually related to the structure of specific molecules, the atoms that they are formed from, and the way that those molecules and atoms interact.
Electron tunneling associated with ferritin is different from other types of molecular electron tunneling, because ferritin is able to store electrons for long periods of time, like a tiny rechargeable battery.
Ferritin’s unique structure — a self-assembled protein shell encapsulating iron — facilitates the conversion of soluble iron into insoluble iron oxide nanoparticles, often referred to as SPIONs. This structure is shown below:
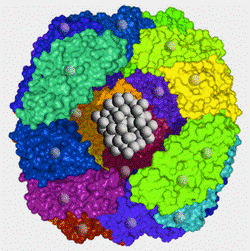
Figure showing ferritin structure with proteins in color and ferrihydrite core shown as grey metallic balls. Electrons can effectively be stored in the ferrihydrite, like a battery. The actual structure of the proteins and of the ferrihydrite and other materials in the core is very complex. Picture from https://mriquestions.com/ferritinhemosiderin.html
Experiments have shown that this structure allows electrons to be stored in the core for hours at a time under certain conditions, which means that the protein shell is able to prevent the electrons from being conducted out of the core under those conditions.
But are electrons able to tunnel into and out of the core? Extensive research by physicists, chemists, and electrical engineers has shown that they can, at least under the right conditions, such as in air or water at room temperature.
Most biologists are familiar with ferritin’s iron storage function but may be unaware of the evidence demonstrating that it can store electrons for hours, and that electrons can tunnel into and out of its core, both under the right conditions. As a result, there are not many tests that demonstrate whether electron tunneling occurs in the conditions that exist in cells and tissues.
However, tests that I commissioned at EAG Labs in Silicon Valley, a commercial testing laboratory with extensive relevant experience, provided the first evidence of electron tunneling through ferritin in biological tissues.
Considering that electron tunneling has been harnessed by man in semiconductor devices for decades, it’s plausible that cells evolved uses for this mechanism in ferritin over more than a billion years.
Ferritin’s unique electrical properties may have initially served early single-celled organisms as an electron “buffer.” Iron and free radicals that include reactive oxygen species (ROS) were early environmental stressors to single-celled organisms. By storing iron and buffering electrons, ferritin potentially offered a dual defense mechanism against these threats.
Scientific studies have reported evidence that ferritin behaves like an antioxidant, which is a chemical that neutralizes ROS in cells, and that antioxidants like vitamin C can donate electrons to ferritin. Ferritin is also overexpressed by the immune system in response to inflammation — a condition marked by ROS.
While circumstantial evidence suggests ferritin-stored electrons can neutralize ROS, a lack of funding hinders dedicated research, such as determining whether that function is aided by electron tunneling. Unfortunately, the challenges of securing funding for quantum biology research often deter scientists from pursuing this promising avenue.
A number of biological processes have previously been identified that appear to utilize electron tunneling associated with ferritin. While there are likely more that have not been identified yet, electron tunneling in ferritin may be a factor for at least cancer, multiple sclerosis, atrial fibrillation, macular degeneration and Parkinson’s Disease, and possibly other diseases and disorders.
Cancer
Cancer cells generate ROS and require efficient neutralization of ROS for survival. Biological immune systems counter ROS by deploying cells called macrophages, which appear to be able to deliver ferritin to ROS-burdened cells. Some types of cancer utilize that immune response mechanism.
While macrophages possess numerous complex functions, their role in ferritin transport is well-established. Many cells produce ferritin, but macrophages appear to be able to supply it to cells to increase the amount of ferritin in the cell more rapidly than the cell could make it on its own.
Experimental evidence also indicates that ferritin in macrophages can conduct electrons over long cellular distances, which could allow macrophages to also supply electrons to ferritin in cancer cells to help neutralize ROS. To definitively confirm whether macrophage-mediated ferritin delivery and subsequent electron transfer to cancer cells is used by cancer for ROS neutralization, targeted research is essential.
Unfortunately, while the research would not be expensive or require special equipment, it remains unexplored due to funding challenges and prevailing biases against quantum biology. If that mechanism is present but is not understood, then that incomplete knowledge could impede or delay finding effective treatments and cures for cancer.
Multiple sclerosis (MS)
MS is an autoimmune disease targeting myelinated neurons. Myelin is a specialized material that is wrapped around axons and dendrites by ferritin-rich cells called oligodendrocytes (in the brain) and Schwann cells (outside the brain). It is essential for efficient neural signaling.
Even though research established decades ago that ferritin is present in myelin, it is rarely considered as a potential actor in myelin research, highlighting the challenges of knowledge dissemination within the vast scientific landscape. Ferritin’s electrical properties could contribute to the unique behavior of myelinated neurons, known as saltatory conduction, but no research has been conducted to investigate that.
The body’s immune response often involves increased ferritin production and delivery, raising the possibility of ferritin-related disruptions in MS. Further research into ferritin’s electrical properties within myelin could shed light on MS pathology. While this research demands specialized equipment, its execution is relatively straightforward and could yield significant insights.
Atrial Fibrillation
Similar to MS, electron conduction through ferritin might contribute to the irregular heart rhythms characteristic of atrial fibrillation (AF).
AF is a potentially fatal condition, and often stems from inflammatory processes that lead to macrophage accumulation. However, resident macrophages in cardiac tissue that are normally present have been shown to contribute to electrical activity in the heart. Additional macrophages that result from inflammation in those tissues could deliver ferritin, disrupting the heart’s electrical circuitry.
Investigating ferritin’s role in AF could thus also be important to a better understanding of atrial fibrillation and to finding effective treatments and cures. While requiring specialized equipment, such research is relatively uncomplicated and could provide valuable insights into AF pathogenesis”
