InflammAging and sclerosis/artritis similaries and differences
2024-08-14
Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis
https://www.mdpi.com/2073-4409/10/12/3402
”
“Systemic sclerosis (SSc) is a chronic connective tissue disorder characterized by immune dysregulation, chronic inflammation, vascular endothelial cell dysfunction, and progressive tissue fibrosis of the skin and internal organs. Moreover, increased cancer incidence and accelerated aging are also found. The increased cancer incidence is believed to be a result of chromosome instability. Accelerated cellular senescence has been confirmed by the shortening of telomere length due to increased DNA breakage, abnormal DNA repair response, and telomerase deficiency mediated by enhanced oxidative/nitrative stresses. The immune dysfunctions of SSc patients are manifested by excessive production of proinflammatory cytokines IL-1, IL-6, IL-17, IFN-α, and TNF-α, which can elicit potent tissue inflammation followed by tissue fibrosis. Furthermore, a number of autoantibodies including anti-topoisomerase 1 (anti-TOPO-1), anti-centromere (ACA or anti-CENP-B), anti-RNA polymerase enzyme (anti-RNAP III), anti-ribonuclear proteins (anti-U1, U2, and U11/U12 RNP), anti-nucleolar antigens (anti-Th/T0, anti-NOR90, anti-Ku, anti-RuvBL1/2, and anti-PM/Scl), and anti-telomere-associated proteins were also found. Based on these data, inflamm-aging caused by immune dysfunction-mediated inflammation exists in patients with SSc. Hence, increased cellular senescence is elicited by the interactions among excessive oxidative stress, pro-inflammatory cytokines, and autoantibodies. In the present review, we will discuss in detail the molecular basis of chromosome instability, increased oxidative stress, and functional adaptation by deranged immunome, which are related to inflamm-aging in patients with SSc”.
What kinds of all kinds of sclerosis are existing?
https://www.mssociety.org.uk/about-ms/what-is-ms
“’Sclerosis’ means scarring and refers to the scars (also called lesions) that MS causes in your brain or spinal cord. These show up in magnetic resonance imaging (MRI) scans. It’s ’multiple’ sclerosis because the lesions happen in more than one place.”
BvS – A problem is that it is written very MUCH about MS but not of ALS and its shared dysfunction, which can be read between the text lines. What we need to focus much on is how biologically sclerosis any where develops while health tissue is slowly (?) gradually change into dysfunctional, probably partly related to dysfunctions of mitochondria (local or general) as well as differential diagnoses hypotheses, e.g. see below
Which Diseases Are Similar to MS?
https://www.healthcentral.com/condition/multiple-sclerosis/diseases-similar-to-ms
2022-03-08
Inflammaging: Age and Systemic, Cellular, and Nuclear Inflammatory Biology in Older Adults https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6777092/ “STAT signal transducer and activator of transcription … Within a community-dwelling sample of older adults, older age is associated with increases in STAT activation, along with increases of systemic inflammatory cytokines. In older adults, heterogeneity in age-related increases in inflammatory disease risk may be related to individual variability in inflammation”
Age-related cerebral small vessel disease and Inflammaging https://www.nature.com/articles/s41419-020-03137-x
“Circulating biomarkers of CSVD (cerebral small vessel diseases) inflammation were classified as markers of systemic inflammation and markers of vascular inflammation/endothelial dysfunction6. Besides, four core MRI features have been identified as imaging markers of CSVD, namely white matter hyperintensities (WMH), lacunae, cerebral microhemorrhage (CMB), and perivascular space enlargement (EPVS)56.” … The regional analysis showed blood markers of vascular inflammation are often associated with deep perforating arteriopathy (DPA), while blood markers of systemic inflammation appear to be associated with cerebral amyloid angiopathy (CAA). Here, we discuss recent findings in the pathophysiology of inflammaging and their effects on the development of age-related CSVD. Furthermore, we speculate the inflammaging as a potential target for future therapeutic interventions to delay or prevent the progression of the age-related CSVD.
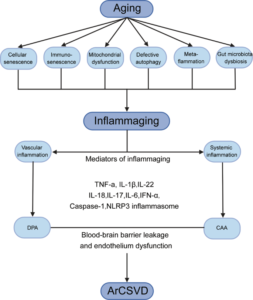
Fig. 1: The deep interactions between aging, inflammaging, and age-related CSVD.
As aging, several cellular and molecular mechanisms lead to chronic inappropriate activation of the immune system. This complex interaction between genetic susceptibility and risk stimuli (both exogenous and endogenous) contributes to the continuous activation of a limited range of confounding sensors which triggers inflammaging (upper part of the box). The resulting synthesis and release of different inflammatory mediators are related to the common pathophysiological mechanisms of age-related diseases. For age-related CSVD, regional analyses showed that blood markers of vascular inflammation were associated with deep perforating arteriopathy (DPA), while blood markers of systemic inflammation were associated with cerebral amyloid angiopathy (CAA), both of which were closely related to the critical pathophysiological mechanisms of blood-brain barrier leakage and endothelial dysfunction (lower part of the box).”
InflammAging & Tinnitus
Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000307 “…This excitatory-to-inhibitory synaptic imbalance was completely prevented by pharmacological blockade of TNF-α expression. These results implicate neuroinflammation as a therapeutic target for treating tinnitus and other hearing loss–related disorders.”
Chronic Inflammation – Inflammaging – in the Ageing Cochlea: A Novel Target for Future Presbycusis Therapy https://www.researchgate.net/publication/320354252_Chronic_Inflammation_-_Inflammaging_-_in_the_Ageing_Cochlea_A_Novel_Target_for_Future_Presbycusis_Therapy
Chronic myelitis:Inflammaging
Emerging roles of frailty and inflammaging in risk assessment of age-related chronic diseases in older adults: The intersection between aging biology and personalized medicine – https://www.researchgate.net/publication/273490290_Emerging_roles_of_frailty_and_inflammaging_in_risk_assessment_of_age-related_chronic_diseases_in_older_adults_The_intersection_between_aging_biology_and_personalized_medicine
An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment
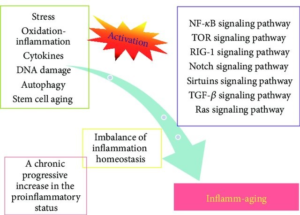
Inflammation in CNS neurodegenerative diseases – https://onlinelibrary.wiley.com/doi/full/10.1111/imm.12922
Inflammation and its resolution and the musculoskeletal system – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5541893/
Anemia at older age: etiologies, clinical implications, and management – https://www.sciencedirect.com/science/article/pii/S0006497120325088
Aging and multiple sclerosis – https://journals.sagepub.com/doi/full/10.1177/1352458516634871 “In this brief literature review, we consider how advancing age influences clinical disease course and pathological and immunological processes implicated in MS. Furthermore, we discuss how several MS symptoms interact with the aging process and advise the clinician how clinical findings may indicate normal aging, relentless MS disease progression, a new disease common in aging, or some combination of these. The optimal differential diagnosis will lead the clinician to the most appropriate therapeutic approach
SE-Inflammation i ryggmärgen
Ansökan om medlemskap i ”Sällsamma diagnoser” https://www.sallsyntadiagnoser.se/wp/wp-content/uploads/2015/06/Ansokan_om_medlemskap_foreningar_och_natverk_aug.-2015.pdf
Kontakt: Felippa Rundgren. E-post: felippa.rundgren@gmail.com (länken nedan)
Transverse myelitis https://www.sallsyntadiagnoser.se/diagnos/transversell-myelit-idiopatisk-myelit-tm/
“Engelskt samlingsnamn för sjukdomen är Transverse Myelitis. TM är en inflammation i ryggmärgen. Orsaken är okänd. Förenklat kan man säga att det skyddande höljet runt nerverna, myelinet, bryts ner och därmed störs/bryts nervsignalerna mellan hjärnan och övriga kroppen”
Gut-Brain
(Old text)
Gut-Brain club
Sökväg till GUT_BTAIN PDF = ”C:\Users\bovon\OneDrive\Dokument\Dokument\AI-Hälsa-Klin-Vet\Klin o icke-klin o verklighet\Klin 2021-2023\OD-Gut-Brain-Maxillaris Inflammaging and Bältros 2023.docx”
Antonio: ” The insights obtained from the present study are expected to provide directions toward designing of microbiome based diagnostic and therapeutic approaches for neurological diseases/disorders”
Concerning Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis
https://www.frontiersin.org/articles/10.3389/fnins.2019.01365/full?fbclid=IwAR24Hw6VRdb9IJfgosfyUhE08NGPbLRIv8t9_QL9xRzqeyRBNFmBmre6AQs or https://www.frontiersin.org/articles/10.3389/fnins.2019.01365/full
Thank you!
Antonio, for forwarding! I do think all health care has to basically change their paradigm from pharmacology medicine reductionism based to Individuals tailored behaviors as food (Like Hippocrates “let medicine be your food and food you medicine) (only as temporary “swim float” for most IF well MOTIVATED and for some unfortunately life jacket for e.g. “no alternative at all) I think Don Moss mentioned a women how was quoted !something like/about” -> Learning in school from the beginning needed a subject that is knowledge and practice of health in general and individuals behaviors in particular.
Good do have others quoted who think in the same way as we concerning basic health care paradigm! While the resistant is “constitutional”, where all focus need to be to SHOW alternative solutions – not only criticizes while this is ignored if observed … would be a collapse if we can not also show HOW IT CAN BE DONE – CHANING GRADUALLY THE PARADIGM! (I am hard working on how it can be done, given interests exists…)
Writing while have hard symptoms (to cope with permanently) – explaining if the above is not very clear
Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis
https://www.frontiersin.org/articles/10.3389/fnins.2019.01365/full?fbclid=IwAR24Hw6VRdb9IJfgosfyUhE08NGPbLRIv8t9_QL9xRzqeyRBNFmBmre6AQs or https://www.frontiersin.org/articles/10.3389/fnins.2019.01365/full
Figure 6. (also in the picture) Schematic representation of the insights obtained from genomic analysis in context of tryptophan metabolism pathways in gut bacteria and their probable connection to brain. The six neuro-active compounds produced through bacterial tryptophan metabolism are depicted using various shapes as shown in the legend provided inside the figure.
For each compound, the top three genera with respect to the “SCORBPEO” value are shown within rectangles having solid borders. For example, bacterial genera such as Klebsiella, Staphylococcus, Ralstonia, etc., produce indole acetic acid (IAA) in the large intestine through tryptophan metabolism. Probable ways by which each of the six compounds may alter the functioning of GBA (as collated from literature) have been indicated by yellow rectangles having dotted border. For instance, IAA may affect the interaction between gut and brain by acting as inter-cellular signaling molecule and immune system modulator. Apart from such mechanisms mediated by tryptophan metabolism products, sequestration of tryptophan by gut bacteria may have a direct impact on brain tryptophan level, which in turn can affect brain function.
Gut-Brain Axis with pictures

https://onlinelibrary.wiley.com/doi/10.1111/jnc.15284
” 1 INTRODUCTION
The reward system is a set of neuronal structures responsible for the processing of a number of psychological components, such as ‘wanting’, ‘liking’ and associative learning (Berridge & Robinson, 2003). These three processes occur together, ‘wanting’ dominates the initial appetitive phase, ‘liking’ dominates the consummatory phase, and learning occurs through the reward-behavioural cycle (Berridge et al., 2016). Both phenomena are underpinned by different brain circuits and neurotransmitter systems. The ‘wanting’ component is largely controlled by the dopaminergic system, whereas the ‘liking’ component is thought to be more mediated by opioid and GABAergic systems (Berridge & Robinson, 2003). The neural circuitry constituting the anatomical and neurochemical substrate for reward and pleasure was described several decades ago by Olds and Milner, who, after implanting electrodes in certain areas of the central nervous system (CNS) and administering electrical microstimuli, demonstrated a pleasure response in the animals (Olds and Milner 1954). Following this, it was proposed that the monoaminergic activity could underlie the neurochemical basis of pleasure, with the monoaminergic system playing an important role in drug reinforcement (Arbuthnott et al., 1970; Fibiger, 1978; Poschel & Ninteman, 1963). This system connects the ventral part of the midbrain with the ventral and anterior part of the brain. One of its best characterized pathways is the mesocorticolimbic projection, especially the dopaminergic pathway (Ikemoto 2010).
The reward pathway is initiated by the activation of dopaminergic neurons located in the ventral tegmental area (VTA), which project to the nucleus accumbens (NAc), the prefrontal cortex (PFC) and the amygdala, where dopamine (DA), together with other neurotransmitters, are released promoting learning and the sensation of pleasure (Bardo, 1998). Another brain structure important role in reward processing is the habenula. This small structure receives inputs from the limbic system and send signals to the VTA, regulating DA levels in the striatal region playing an important role in reward and reward-associated learning (Baker et al., 2016; Proulx et al., 2014; Velasquez et al., 2014). Drugs of abuse (non-natural stimuli) have the ability to induce DA release of greater amplitude and duration than those induced by natural rewards (Wise & Rompre, 1989). Despite the capacity of non-natural rewards to hijack the brain circuits in which natural rewards act, a high degree of overlap has been observed in the brain regions that process both stimuli (Olsen, 2011). Plasticity in these circuits may be responsible for behavioural alterations (affecting motivation, executive function and reward processing) characteristic of addiction and dependence (Kalivas & O’Brien, 2008; Kelley & Berridge, 2002; Koob & Volkow, 2010).
There has been an increasing emphasis on the contribution of factors outside the brain in regulating central processes. Indeed, a growing body of research focuses on the ability of both the endocrine and the immune system on modulating reward processes (Adam & Epel, 2007; Anisman et al., 1996; Goeders, 2002; Greene et al., 2019; Montesinos et al., 2016; Ye et al., 2001). Over the past decade a new player has emerged as another key factor in sculpting reward circuits across the lifespan, namely, the gut microbiome, which encompasses the trillions of bacteria in our gut. This review will focus on the emerging data that demonstrate how gut microbes may be involved in regulating brain reward functions influencing feeding, social and addictive behaviours (Figure 1). We will first provide a brief overview of microbiota-brain connections explaining the main pathways of communication. Then, we will discuss the role of gut microbiota as a regulator of the reward system integrating new preclinical and clinical evidence on both natural and non-natural rewards. We will describe potential mechanisms of action and the implications for the development of psychiatric disorders. Finally, we will examine the emergent evidence of microbiome-driven interventions and its therapeutic effects in psychiatric disorders associated with reward alterations.”

FIGURE 1
Potential pathways by which the intestinal microbiota influences brain reward processes. The main communication pathways of the microbiome-gut-brain axis are the vagus nerve, the immune system, HPA axis, bacterial metabolites and enteroendocrine cells. Gut microbes are able to produce various metabolites, such as neurotransmitters or neuroactive compounds, SCFAs, mimetic peptide sequences and toxins. These metabolites act on enteroendocrine cells, the vagus nerve or by translocation throughout the gut epithelium into the systemic circulation. Neurotransmitters and neuroactive peptides produced or altered by the gut microbiota can be released to the systemic circulation and may have an impact on host physiology altering the reward system. Stimulation of enteroendocrine cells by SCFAs release gut hormones such as CCK, GLP-1 and PYY, and can increase the concentration of peripheral hormones such as ghrelin, leptin and insulin targeting different areas on the brain reward system. These peripheral hormones can be affected by auto-antibodies interacting with microbial mimetic peptides as ClpB. Alterations in the gut epithelium (‘leaky gut’) has an impact in the presence of gut microbial toxins such as LPS. This molecule activates the immune response (IL, TNF) crossing the blood–brain barrier or activating the vagus nerve altering brain reward functioning. Abbreviations: PFC prefrontal cortex, Nac nucleus accumbens, VTA ventral tegmental area, CCK cholecystokinin, GLP-1 glucagon-like peptide, PYY peptide YY, IL interleukin, TNF tumour necrosis factor, ClpB caseinolytic protease B, LPS Lipopolysaccharide
2 THE MICROBIOTA-GUT BRAIN AXIS
By definition, the microbiome is considered a community of microorganisms—the bacteria, fungi, virus and other single cell microorganisms living together in a particular habitat (Ursell et al., 2012). One of the most exciting advances in biology has been the understanding of how the microbiome influences host physiology and behaviour (Cryan & Dinan, 2012; Gilbert et al., 2018). Not only do humans, animals and plants have their own unique microbiome (Butler et al. 2019; Compant et al., 2019; Knight et al., 2017); but soils, forests and oceans do as well (Baldrian, 2016; Lakshmanan et al., 2014; Sunagawa et al., 2015), which demonstrates the importance of microorganisms in the overall functioning of life. The human body harbours about 100 billions microbes, outnumbering the human cells in the body by a ratio of 1.3 to 1 (Sender et al., 2016). The majority of these microorganisms are found in the gastrointestinal tract, resulting in a complex symbiotic relationship that is acquired immediately at birth (Robertson et al., 2019; Tamburini et al., 2016). Although there has been some reports of existence of microbiota in the placenta and in utero colonization in the foetus (Aagaard et al., 2014; Collado et al., 2016; Jiménez et al., 2008), these findings have been disputed by others suggest limited evidence by a lack of contamination controls, maintaining the idea that the placenta and womb are sterile (Lauder et al., 2016; Lim et al., 2019; Milani et al., 2017; Perez-Muñoz et al., 2017; Rackaityte et al., 2020). The gut is densely populated with greater diversity of microorganisms that any other organ. The interaction between the microbiota and the host is intricate, creating a mutually beneficial relationship. On the one hand, the microbiota has the ability to contribute to a multitude of physiological processes such as protection against pathogens, modulation of the immune system, fermentation of undigestible dietary fibres and production of active peptides and proteins that regulate energy metabolism in the host (Kennedy et al., 2017; Mayer et al., 2015; Rooks & Garrett, 2016). On the other hand, the host provides protection and nutrients necessary for the development of the microbiological ecosystem (Foster et al., 2017; Mushegian & Ebert, 2016), highlighting the mutualistic relationship between the host and the microbiome.
Although a basic understanding of the interaction between the microbiota and the host dates back to ancient times, recently, the interest on the impact of intestinal microbiota on health and disease has re-emerged. This has largely been because of advances in sequencing technologies coupled with the appropriated bioinformatic tools (Gevers et al., 2012; Hao et al., 2017). Moreover there has been a growing awareness of the crosstalk between our intestinal bacteria, the CNS and behaviour. The microbiota-gut-brain-axis is a bidirectional pathway throughout which the brain regulates the activity of the gut and vice versa and is critical for the homeostasis of the host system (Rhee et al., 2009; Collins et al., 2012; Cryan et al. 2019) (Figure 1). Signals from the gastrointestinal tract to the brain are able to influence a number of brain functions, and the brain responds by executing decisions that determine seeking, intake and behaviours (eating, social, activity), which shows that both organs are constantly communicating and influencing each other. Communication in the microbiota-gut-brain axis does not only depend on neuronal signals (neurotransmitters), it also depends on endocrine (hormones and gut peptides) and immune signals (cytokines), and microbiota derived metabolites, acting together to regulate host physiology and microbiota composition.
2.1 The vagus nerve
The vagus nerve is one of the major modulatory constitutive communication pathways between the microbiota and the brain (Fülling et al., 2019). The vagal afferent nerves are in contact with all the layers of the digestive wall, but do not cross the intestinal epithelial barrier so they are not in direct contact with the intestinal microbiota (Bonaz et al., 2018). Thus, these nerve fibres can respond only to indirect microbial signals, such as neurotransmitters or short chain-fatty acids (SCFA), which will diffuse through the epithelial walls or through interaction with enteroendocrine cells (EECs) (Raybould, 2010; Ye & Liddle, 2016). It has also been described that gastrointestinal hormones such as cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), peptide YY (PYY) or ghrelin can act on the CNS through the vagus, thanks to the presence of specific receptors on these fibres (Ye & Liddle, 2016). Evidence suggests that the microbiota has the ability to alter host behaviours throughout the vagus nerve, transducing beneficial microbial-derived effects (Bravo et al., 2011; Bercik et al., 2011; Vazquez et al., 2016; Sgritta et al., 2019), and modulating anxiety-related behaviours (Goehler et al., 2005).
2.2 Immune system
Another important role of the microbiota is its contribution as an immune system modulator. The gastrointestinal tract represents the major interface between the immune system and the microorganisms, and the ability to discriminate commensal from pathogens can be considered as the driving force of immune system evolution (Mezouar et al., 2018). Cytokines (key mediators of immune system function) can signal to the brain indirectly via the vagus nerve or directly throughout the blood–brain barrier (BBB). Lipopolysaccharide (LPS), the largest component of the external membrane of Gram-negative bacteria, plays an important role in activating the immune system as it comprises the most important surface antigen of this type of bacteria. This component present in the cell wall activates toll-like receptors (TLRs), which are widely expressed in immune cells. Once these receptors are activated, they can recruit inflammatory mediators, cause cytokine production and chemokine-mediated recruitment of acute inflammatory cells (Daulatzai, 2014). Released cytokines can act on the CNS via slow transmission, involving cytokines produced by different tissues and diffusing into the brain (Banks, 2008) or via fast transmission, involving afferent nerves innervating the body and signalling the brain (Goehler et al., 2005). The activation of the brain cytokine system is associated with changes in sickness behaviour altering sleep, sociability, eating, cognition and motivation (Konsman et al., 2002). For example, the administration of the VSL#3 (composed of various Lactobacilli, Bifidobacteria and Streptococcus) attenuated sickness behaviours and inflammation, demonstrating that the microbiota is able to modulate inflammatory responses, which can affect brain function and behaviour (D’Mello et al., 2015). The gut microbiota has also been implicated in microglial maturation and function (Erny et al., 2015), or the increased neuroinflammatory responses at middle age and at advanced age (inflammaging) (Boehme et al., 2020; Thevaranjan et al., 2017; Xia et al., 2016), reinforcing the idea of the microbiome as an essential modulator of a healthy immune response.
2.3 Bacterial metabolites: Short-chain fatty acids and neurotransmitters
Under anaerobic conditions, undigested carbohydrates are fermented mainly into short-chain fatty acids (SCFA), the most extensively studied molecules with respect to how the gut microbiota influence host physiology. These molecules have multiple effects in the host, principally as an energy source (Dalile et al., 2019). It has also been demonstrated that SCFA can elicit intracellular signalling by binding to G-protein-coupled receptors (e.g. free fatty acid receptors or FFARs). These receptors are involved in controlling anorexigenic hormones (i.e. PYY and GLP-1), providing a potential link between SCFA and appetite control (Byrne et al., 2015). Recently, it was also shown that SCFA can attenuate ghrelin receptor signalling (Torres-Fuentes et al., 2019), a key receptor at the interface of appetite regulation and food reward (Schellekens et al., 2012). In addition, microbial-derived SCFAs can also influence brain physiology and behaviour directly. Circulating SCFAs, such as butyrate, acetate and propionate, are able to cross the BBB throughout monocarboxylate transporters (Maurer et al., 2004), and enter the CNS to exert their effects on neurons and glial cells (Joseph et al., 2017). Systemic administration of sodium butyrate has also been reported to induce transient acetylation of histones in the frontal cortex and hippocampus, inducing antidepressant-like effects in mice (Schroeder et al., 2007). Evidence suggests that SCFAs are capable of modulating neurotransmission (DeCastro et al., 2005; Nankova et al., 2014; Shah et al., 2006), for example increasing the expression of tyrosine hydroxylase and decreasing the expression of dopamine-β-hydroxylase (DeCastro et al., 2005; Mally et al., 2004). Also, it has been observed that propionic acid modulates serotoninergic, GABAergic and dopaminergic neurotransmission in vivo (El-Ansary et al., 2012). In particular, the hippocampus and striatum—crucial areas for reward behaviours—seem to be influenced by gut-derived SCFAs (van Byrne et al., 2015; de Wouw et al., 2018).
The microbiota has been shown to synthesize and respond to several neurochemical compounds associated with the host mood and behaviour (i.e. DA, serotonin (5-HT), γ-aminobutyric acid (GABA), etc) (Strandwitz, 2018; Wall et al., 2014). These neuroactive compounds can enter into the circulatory system reaching distal areas such as the brain. Therefore, the gut-mediated regulation of these different neurotransmitters may have a crucial role in brain processes. Catecholamine levels have been shown to be altered in germ-free mice when compared with specific pathogen free, suggesting the ability of the gut microbiota to produce these compounds (Asano et al., 2012). However, the possibility that these catecholamines can act distally in the brain is highly questionable, since they are not able to cross the BBB. In spite of this, there is evidence suggesting that the gut microbiota is capable of inducing changes at the catecholaminergic level in the brain. A clear example has been observed in germ-free animals, which showed a decrease in the amino acid tyrosine (essential amino acid for the synthesis of noradrenaline (NA) and DA) when compared to recolonized germ-free mice, which induced an increase in the brain DA levels (Matsumoto et al., 2013). Some gut microbes are also able to metabolize tryptophan (the precursor of 5-HT). It has been observed in germ-free mice a reduction 5-HT levels in plasma, colon and caecum (Clarke et al., 2013; Hata et al., 2017; Wikoff et al., 2009), and the conventionalization of germ-free mice resulted in an increase of intestinal 5-HT. Indeed, bacterial species such as Clostridium perfringens modulates host colonic 5-HT synthesis via host tryptophan hydroxylase, highlighting the role for the host-microbiota in regulating 5-HT-related biological processes (Yano et al., 2015). Gut microbiota is also able to covert the amino acid glutamate into GABA. Interestingly, certain Escherichia, Lactobacillus and Bifidobacterium have been shown to synthesize GABA. Gut-derived GABA, unlike catecholamines, is able to reach the CNS because of the presence of specific GABA transporters expressed in the BBB (Takanaga et al., 2001).
2.4 Enteroendocrine signaling
EECs are specialized cells distributed throughout the gastrointestinal tract and represent approximately 1% of the epithelial cells (Latorre et al., 2016). EEC secretory products such as CCK, GLP-1 and PYY are released in response to diverse stimuli to influence physiological functions in the host such as control of intestinal secretion and motility, regulation of food intake and metabolism (Latorre et al., 2016; Rehfeld, 2004). Hormones and gut peptides secreted by EECs act on receptors present on the vagus nerve that bring stimuli to the brain and may mediate satiety and other behavioural responses. However, it is unclear whether some of these peptides interact directly with the brain or how the brain interprets the information coming from the gut. Recently, a new route of communication between EECs and the enteric nervous system has been described (Bohórquez et al., 2014, 2015; Kaelberer et al., 2018). A basolateral elongation, called neuropod, has been observed in some of the EECs, and is capable of establishing a functional synapse with enteric glia and vagal afferents (Kaelberer et al., 2018, 2020; Liddle, 2019). This interesting connection suggests that the intestinal signals from the EECs to the brain may be more precise and faster than expected, and that the CNS may have the ability to interact with the EECs through these connections. Additionally, receptors for gut peptides have been described in central brain regions such as hypothalamus and nucleus tractus solitarii (NTS), indicating that these peptides might modulate their responses acting directly in the brain (Ballaz, 2017; Dourish et al., 1989; Göke et al., 1995; Ohkubo et al., 1990; van Bloemendaal et al., 2014). Although inconsistencies exist, different microbiome-targeted studies have demonstrated that the microbiota modulates EECs signalling (Covasa et al., 2019; Plovier & Cani, 2017). Preliminary evidence demonstrates that germ-free animals exhibit attenuation of gut peptides expression, suggesting that the microbiota may be responsible for stimulation and production of satiety peptides (Duca et al., 2012). Furthermore, the administration of oligofructose and inulin (dietary fructans) promote satiety in rats, increasing GLP-1 and decreasing ghrelin in the gut (Cani et al., 2004), indicating that modulations of the gut bacteria regulates gut peptides. Moreover the existing association between microbiome changes and ghrelin levels were recently highlighted (Schalla & Stengel, 2020). In theory, SCFAs may have the capability to modulate these peptides, through their interactions with FFAR receptors present in EECs promoting the release of gut hormones (Nøhr et al., 2013).
2.5 Hypothalamic-pituitary-adrenal axis (HPA)
The HPA axis is a complex set of pathways and interactions among the hypothalamus, the pituitary gland and the adrenal glands. Neurons in the hypothalamus release corticotrophin-releasing factor and vasopressin stimulating the release of the adrenocorticotrophic hormone in the pituitary gland which in turn promotes the release of glucocorticoid hormones from the adrenal glands (Besedovsky et al., 1991). The HPA axis has an important role regulating homeostatic systems in the body (i.e. CNS, immune system, metabolic system, reproductive system, mood and emotion) and is the main coordinator of stress response. It has been observed that different types of stress can alter the composition of the gut microbiota (Bailey, 2014; Bharwani et al., 2016; De Palma et al. 2014), but also gut microbiota can influence HPA responses (Frankiensztajn et al., 2020; Wall et al., 2014). Studies in germ-free animals demonstrated an exaggerated HPA response (elevated adrenocorticotrophic hormone and corticosterone) when exposed to stressors (Clarke et al., 2013; Sudo et al., 2004), suggesting the relationship between the gut microbiota and stress altering HPA axis. Gut microbiota seems to activate HPA axis through modulations of the immune system or microbial metabolites (i.e. SCFAs or neurotransmitters) inducing a dysfunctional response to the host and contributing to the development of CNS disorders (Moloney et al., 2014; Rea et al., 2016).
3 THE MICROBIOTA AS A REGULATOR OF THE REWARD PATHWAY
It is well known that microbes resident in the gastrointestinal tract are capable of influencing host functions and behaviours, demonstrating that the host and its symbiotic microbes can behave as one evolutionary entity called holobiont (Margulis & Fester, 1991). This close interaction between the host and the microbiome has provided a clear evolutionary advantage that has favoured survival and fitness for both. Such microbiota-driven alterations in host behaviour have led to the hypothesis that some symbionts manipulate the host for their own purposes (Johnson & Foster, 2018; Sampson et al., 2016). For example, some authors suggest that microorganisms could influence individual´s nutritional aspects modifying feeding decisions or even making us more sociable to promote contact between hosts and increase their own transmission (Alcock et al., 2014; Leitão-Gonçalves et al., 2017). Behavioural changes induced by microbes have also been traced to specific subsets of the microbiota. Evidence suggests that Lactobacillus and Bifidobacterium species can relieve anxiety and depressive symptoms in animals and humans (Aizawa et al., 2016; Desbonnet et al., 2008; Jang et al., 2018). In addition, Bacteroides species have been shown to improve repetitive and anxious behaviours and communication deficiencies in mice, purportedly by restoring specific bacterial metabolites (i.e. 4-ethylphenylsulfate and indolepyruvate) (Hsiao et al., 2013). These microbiome-induced alterations show that they could play a role in regulating one of the most important and oldest systems in our brain, the reward system (Figure 1). Growing evidence indicates that perturbations in the microbiota can influence monoaminergic neurotransmission (i.e. dopaminergic neurotransmission) in the mesocorticolimbic circuit, which is involved in natural and non-natural rewards (González-Arancibia et al., 2019). Such perturbations also appear to be a signature in psychiatric disorders in which the mesocorticolimbic circuit is highly involved (Skosnik & Cortes-Briones, 2016).
4 NATURAL REWARDS
4.1 The microbiota as a regulator of food reward
See more at the link ..
Gut bacteria may hold the key to preventing Parkinson’s disease, say Finnish scientists
Evolution of inflammation, specially gut-brain evolution
2024-07-14
Tracing the Evolutionary Origin of the Gut–Brain Axis
https://www.researchgate.net/publication/327255475_Tracing_the_Evolutionary_Origin_of_the_Gut-Brain_Axis
Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health https://www.mdpi.com/2072-6643/13/1/196
“The relatively rapid shift from consuming preagricultural wild foods for thousands of years, to consuming postindustrial semi-processed and ultra-processed foods endemic of the Western world less than 200 years ago did not allow for evolutionary adaptation of the commensal microbial species that inhabit the human gastrointestinal (GI) tract, and this has significantly impacted gut health. The human gut microbiota, the diverse and dynamic population of microbes, has been demonstrated to have extensive and important interactions with the digestive, immune, and nervous systems. Western diet-induced dysbiosis of the gut microbiota has been shown to negatively impact human digestive physiology, to have pathogenic effects on the immune system, and, in turn, cause exaggerated neuroinflammation. Given the tremendous amount of evidence linking neuroinflammation with neural dysfunction, it is no surprise that the Western diet has been implicated in the development of many diseases and disorders of the brain, including memory impairments, neurodegenerative disorders, and depression. In this review, we discuss each of these concepts to understand how what we eat can lead to cognitive and psychiatric diseases.”
Early life intestinal inflammation alters gut microbiome, impairing gut-brain communication and reproductive behavior in mice https://www.biorxiv.org/content/10.1101/2024.05.23.595618v1
Below just moved to (will be shortly removed from www.carism.se)
https://www.boaim2.se/complex-diseases/inflammaging-huvud/inflammaging/inflammaging-and-quantum-behaviors-and-more-material-to-elaborate/
InflammAging
https://www.researchgate.net/publication/262386439_Chronic_Inflammation_Inflammaging_and_Its_Potential_Contribution_to_Age-Associated_Diseases
InflammAging and molecular biology
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/inflammaging
InflammAging, cytokines, …
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8834134/
Quantum michrocondria
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5264502/
InflammAging and Quantum – Thermodynamics and Inflammation: Insights into Quantum Biology and Ageing https://www.mdpi.com/2624-960X/4/1/5
”Abstract: Inflammation as a biological concept has been around a long time and derives from the Latin “to set on fire” and refers to the redness and heat, and usually swelling, which accompanies injury and infection. Chronic inflammation is also associated with ageing and is described by the term “inflammaging”. Likewise, the biological concept of hormesis, in the guise of what “does not kill you, makes you stronger”, has long been recognized, but in contrast, seems to have anti-inflammatory and age-slowing characteristics. As both phenomena act to restore homeostasis, they may share some common underlying principles. Thermodynamics describes the relationship between heat and energy, but is also intimately related to quantum mechanics. Life can be viewed as a series of self-renewing dissipative structures existing far from equilibrium as vortexes of “negentropy” that ages and dies; but, through reproduction and speciation, new robust structures are created, enabling life to adapt and continue in response to ever changing environments. In short, life can be viewed as a natural consequence of thermodynamics to dissipate energy to restore equilibrium; each component of this system is replaceable. However, at the molecular level, there is perhaps a deeper question: is life dependent on, or has it enhanced, quantum effects in space and time beyond those normally expected at the atomistic scale and temperatures that life operates at? There is some evidence it has. Certainly, the dissipative adaptive mechanism described by thermodynamics is now being extended into the quantum realm. Fascinating though this topic is, does exploring the relationship between quantum mechanics, thermodynamics, and biology give us a greater insight into ageing and, thus, medicine? It could be said that hormesis and inflammation are expressions of thermodynamic and quantum principles that control ageing via natural selection that could operate at all scales of life. Inflammation could be viewed as a mechanism to remove inefficient systems in response to stress to enable rebuilding of more functional dissipative structures, and hormesis as the process describing the ability to adapt; underlying this is the manipulation of fundamental quantum principles. Defining what “quantum biological normality” is has been a long-term problem, but perhaps we do not need to, as it is simply an expression of one end of the normal quantum mechanical spectrum, implying that biology could inform us as to how we can define the quantum world.”
Inflammation at cell levels
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5805548/
Cold-InflammAging …
https://onlinelibrary.wiley.com/doi/10.1111/1440-1681.13686
Testosteron decrease in Inflammaging
https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2024.1291389/full
Immunosenescence: molecular mechanisms and diseases | SignalTransduction and Targeted Therapy https://www.nature.com/articles/s41392-023-01451-2
(Inflammaging….
Inflammation and aging: signaling pathways and interventiontherapies | Signal Transduction and Targeted Therapy
https://www.nature.com/articles/s41392-023-01502-8
”Also influence coherence in enzymatic functions?
”“Aging is characterized by systemic chronic inflammation, which is accompanied by cellular senescence, immunosenescence, organ dysfunction, and age-related diseases. Given the multidimensional complexity of aging, there is an urgent need for a systematic organization of inflammaging through dimensionality reduction. Factors secreted by senescent cells, known as the senescence-associated secretory phenotype (SASP), promote chronic inflammation and can induce senescence in normal cells. At the same time, chronic inflammation accelerates the senescence of immune cells, resulting in weakened immune function and an inability to clear senescent cells and inflammatory factors, which creates a vicious cycle of inflammation and senescence. Persistently elevated inflammation levels in organs such as the bone marrow, liver, and lungs cannot be eliminated in time, leading to organ damage and aging-related diseases. Therefore, inflammation has been recognized as an endogenous factor in aging, and the elimination of inflammation could be a potential strategy for anti-aging. Here we discuss inflammaging at the molecular, cellular, organ, and disease levels, and review current aging models, the implications of cutting-edge single cell technologies, as well as anti-aging strategies. Since preventing and alleviating aging-related diseases and improving the overall quality of life are the ultimate goals of aging research, our review highlights the critical features and potential mechanisms of inflammation and aging, along with the latest developments and future directions in aging research, providing a theoretical foundation for novel and practical anti-aging strategies.”
